

INTELLIGENT ENGINEERING
Diverse Solutions for a Sustainable Energy Future
The worldwide drive for clean power has sparked the growth and development of diverse green fuels, each with unique properties, costs, and environmental impacts. E-methanol offers a greener alternative to traditional methanol in the chemical industry. Green Ammonia presents a sustainable option for fertiliser production. Green Hydrogen shows promise as a low-carbon energy carrier despite production and storage challenges. Biofuels, including Biogas, Bioethanol, and Biodiesel, provide renewable options with varying carbon footprints.
Methanol
Methanol is an important chemical compound with significant industrial applications and various production methods. Methanol is currently usually produced from syngas, obtained from steam reforming of methane. This process requires 30 GJ of energy per tonne, translating to 0.03 GJ/kg, with production costs ranging from $0.1 to $0.25 per kilogram. Methanol’s heat of combustion (LHV) is 20 MJ/kg. It is predominantly used as a chemical building block for numerous products, contributing significantly to the chemical industry.
E-methanol, an alternative to traditional methanol, is produced using green hydrogen and captured carbon dioxide through a chemical reaction. This method demands 36 GJ of energy per ton. Production costs vary significantly. If carbon dioxide is produced from direct air capture, its production costs range from $0.8 to $1.6 per kilogram, and if it is sourced from carbon capture and storage, its production costs increase to 1.2-2.4 $ per kg. E-Methanol boasts lower emissions, less than 10 g CO2 per kg, making it a more environmentally friendly option compared to traditional methanol. However, its current world production is less than 0.2 million metric tonnes per year, indicating its nascent stage in the market.
Methanol is highly relevant in the global market, with a world production of 110 million metric tonnes per year and a demand of 84.77 million metric tonnes per year as of 2022. It has notable environmental impacts, emitting 110 g CO2 per MJ. Despite this, its vast market size and wide range of applications underscore its critical role in the chemical industry and potential for sustainable alternatives like e-Methanol.
The development and adoption of e-methanol are pivotal in reducing the carbon footprint associated with methanol production, provided renewable energy sources are utilised. As industries strive for more sustainable practices, e-methanol presents a promising alternative that aligns with global efforts to mitigate climate change and reduce industrial emissions.
Production Processes
- Traditional Methanol Production: Typically, methanol is produced through the steam reforming of natural gas. This involves the reaction of carbon monoxide (CO) and hydrogen (H₂) to form methanol.
- Energy Requirement: 30 GJ per ton of methanol.
- E-Methanol Production: A greener alternative, e-methanol is produced by combining green hydrogen (H₂) with captured carbon dioxide (CO₂). This method leverages renewable energy sources, significantly lowering the carbon footprint.
- Energy Requirement: Approximately 10-11 MWh of electricity per 1000 kg of e-methanol (equivalent to 0.036 GJ/kg).
Carbon Emissions
- Traditional Methanol: The carbon emissions depend heavily on the energy source used for production. Traditional methods can have substantial CO₂ emissions due to reliance on fossil fuels.
- E-Methanol: Emissions are considerably lower, provided the hydrogen is produced using renewable energy. This makes e-methanol a more sustainable option.
Market Dynamics
- Production Volume: Methanol has a substantial global production volume, with 110 million metric tons produced annually.
- Demand: The annual demand for methanol is approximately 84.77 million metric tons, reflecting its extensive use across various industries.
Applications
Methanol is used in a variety of applications:
- Fuel: Direct use as a fuel in internal combustion engines or as a component in biodiesel.
- Chemical Feedstock: Used in the production of formaldehyde, acetic acid, and other chemicals.
- Energy Storage: Methanol can be utilised in fuel cells for energy storage and generation.
Ammonia
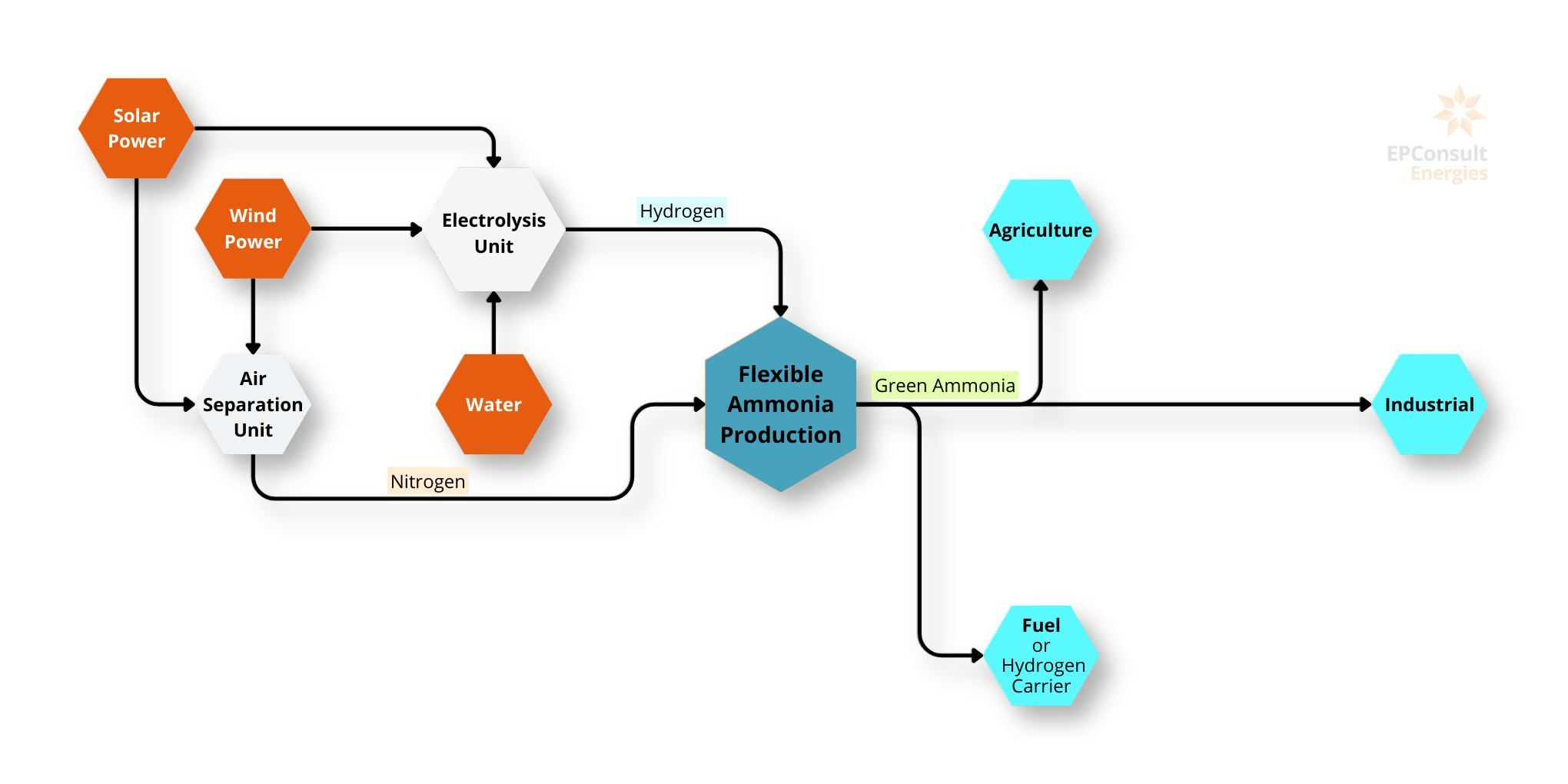
Ammonia (NH3) is a vital chemical in various industrial applications, particularly in agriculture as a key component of fertilisers. The traditional production method for ammonia is the Haber Process, which involves synthesising ammonia from nitrogen and hydrogen (3H2 + N2 => 2NH3). This process is energy-intensive, requiring 0.029 GJ/kg, and the production costs range between $0.1 and $0.35 per kilogram. The heat of combustion (LHV) for ammonia is 19 MJ/kg.
Green Ammonia represents a more sustainable alternative. If hydrogen is produced through the electrolysis of water using renewable energy sources i.e. green hydrogen, and combined with nitrogen, then the process yields in Green Ammonia. This method eliminates carbon emissions during production, making it a promising solution for reducing the environmental impact of ammonia production. The specific energy required to produce green ammonia is basically the same as ammonia, but the production cost is higher: 0.7-1.4 $/kg.
Ammonia production is significant on a global scale, with an annual production of 158 million metric tonnes and a demand of 167 million metric tonnes. The production process, particularly the Haber Process, is responsible for considerable carbon emissions, releasing approximately 1.6 tonnes of CO2 per tonne of ammonia produced. This contributes to about 1.4% of global carbon dioxide emissions and represents 1% of the world's energy consumption.
In contrast, green ammonia, which produces zero CO2 emissions, had a modest production of 0.02 million metric tonnes as of 2021, with a projected demand of 100 million metric tonnes. This highlights the growing interest and potential market expansion for green ammonia as industries aim to transition to more sustainable practices.
The primary use of ammonia is in fertilisers, with about 80% of industrially produced ammonia dedicated to this purpose. Green ammonia, apart from its traditional uses, is also seen as a potential carrier for green hydrogen, offering a dual benefit in both fertiliser production and as a clean energy source.
In summary, while traditional ammonia production is deeply entrenched and critical to global agriculture, its environmental impact necessitates a shift towards greener alternatives. Green ammonia offers a sustainable pathway, aligning with global efforts to reduce carbon emissions and energy consumption.
Production Processes
Traditional Ammonia Production: The primary method for producing ammonia is the Haber Process, which involves synthesising ammonia from nitrogen (N2) and hydrogen (H2).
- Energy Requirement: 6-7 Gcal per ton of ammonia, equivalent to 0.029 GJ/kg.
Green Ammonia Production: A more sustainable method, green ammonia is produced by combining green hydrogen (H2), generated through the electrolysis of water using renewable energy, with nitrogen (N2). This method eliminates carbon emissions during production.
- Energy Requirement: The energy requirement is the same as traditional ammonia.
Carbon Emissions
Traditional Ammonia: The carbon emissions are substantial, with the production process emitting approximately 1.6 tonnes of CO2 per tonne of ammonia produced. This method contributes to 1.4% of global carbon dioxide emissions and represents 1% of global energy consumption.
Green Ammonia: Production of green ammonia results in zero CO2 emissions, provided that renewable energy sources are used for the electrolysis of water to produce green hydrogen.
Market Dynamics
Production Volume: Ammonia has a significant global production volume, with 158 million metric tonnes produced annually.
Demand: The annual demand for ammonia is approximately 167 million metric tonnes, reflecting its extensive use, particularly in agriculture.
Applications
Ammonia is used in a variety of applications:
Fertiliser: About 80% of industrial ammonia is used to produce fertilisers, which are crucial for global agriculture.
Energy Storage and Transportation: Green ammonia is seen as a potential carrier for green hydrogen, offering benefits in both fertiliser production and as a clean energy source.
Industrial Use: Ammonia is also used in the production of explosives, pharmaceuticals, and other chemicals.
Hydrogen
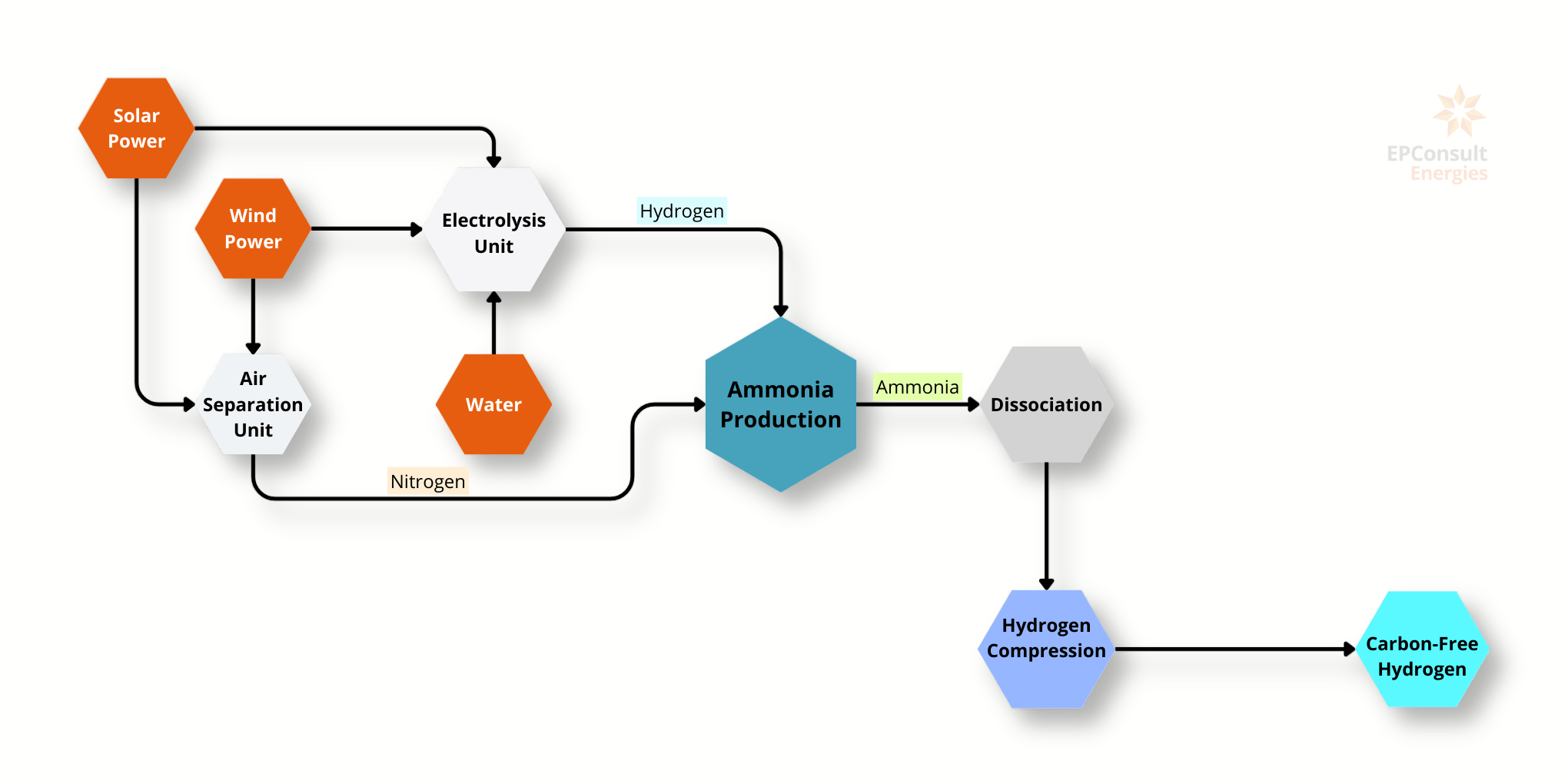
Hydrogen (H2) is a versatile and clean energy carrier with significant potential for various applications, including energy storage, transportation, and industrial uses. Green hydrogen, specifically, is produced through the electrolysis of water using renewable energy sources. This process splits water into hydrogen and oxygen, with a specific energy requirement of 50 kWh per kilogram of hydrogen, which translates to approximately 0.18 GJ/kg. The production costs for green hydrogen range between $3.0 and $6.0 per kilogram.
Hydrogen's heat of combustion (LHV) is 120 MJ/kg, making it a highly efficient energy source. Green hydrogen is characterised by its low to zero carbon emissions, depending on the energy source used for electrolysis. Emissions can range from 0 to 2 tonnes of CO2 per tonne of hydrogen, with the lower end achievable through the exclusive use of renewable energy.
The global production of hydrogen is currently at 0.04 million metric tonnes per year, with a demand of 85 million metric tonnes per year. This disparity highlights the growing interest and need for expanding green hydrogen production to meet increasing demand.
Hydrogen is used in various applications, including powering vehicles, generating electricity, and as a feedstock in industrial processes. Despite its potential, hydrogen storage remains challenging due to its low energy density and the need for high-pressure or cryogenic storage solutions. Recent research in metal hydrides and other advanced storage technologies aims to address these challenges and make hydrogen more viable as a mainstream energy carrier.
In summary, green hydrogen presents a promising pathway towards a sustainable and low-carbon energy future. Its production through renewable energy sources aligns with global efforts to mitigate climate change and reduce dependence on fossil fuels. The development of efficient storage and distribution systems will be crucial in realising hydrogen's full potential.
Production Processes
Green Hydrogen Production: Green hydrogen is produced through the electrolysis of water, which uses electricity from renewable sources to split water into hydrogen (H2) and oxygen (O2).
- Energy Requirement: 50 kWh per kilogram of hydrogen, equivalent to 0.18 GJ/kg.
Carbon Emissions
Green Hydrogen: The carbon emissions associated with green hydrogen production depend on the energy source used for electrolysis. With renewable energy, emissions can be as low as 0 tonnes of CO2 per tonne of hydrogen, but they can reach up to 2 tonnes of CO2 per tonne of hydrogen if non-renewable sources are used.
Market Dynamics
Production Volume: The current global production of hydrogen is 0.04 million metric tonnes per year.
Demand: The annual demand for hydrogen is approximately 85 million metric tonnes, indicating significant potential for market growth.
Applications
Hydrogen is used in various applications:
Transportation: Hydrogen can power fuel cell vehicles, providing a clean alternative to traditional internal combustion engines.
Energy Generation: Hydrogen can be used to generate electricity in fuel cells, offering a sustainable energy solution.
Industrial Use: Hydrogen serves as a feedstock in various refinery and petrochemical processes, including ammonia and methanol production.
Energy Storage: Hydrogen energy storage is a form of chemical energy storage in which electrical power is converted into hydrogen. This energy can then be released again by using the gas as fuel in a combustion engine or a fuel cell.
Challenges and Opportunities
Storage: There are several hydrogen storage techniques, each with advantages and disadvantages. In gaseous hydrogen storage, hydrogen gas is compressed and stored at high pressures, requiring robust and expensive pressure vessels. In liquid hydrogen storage, hydrogen is cooled to extremely low temperatures and stored as a liquid, which is energy-intensive.
Research and Development: Ongoing research in advanced storage technologies, such as metal hydrides, aims to overcome storage challenges and enhance hydrogen's practicality as an energy carrier.
Sustainability: Green hydrogen aligns with global sustainability goals, offering a pathway to reduce carbon emissions and dependency on fossil fuels, provided renewable energy sources are used for its production.
Read more about operation and transport risks HERE
Read our extensive publication about White Hydrogen HERE
Biofuels
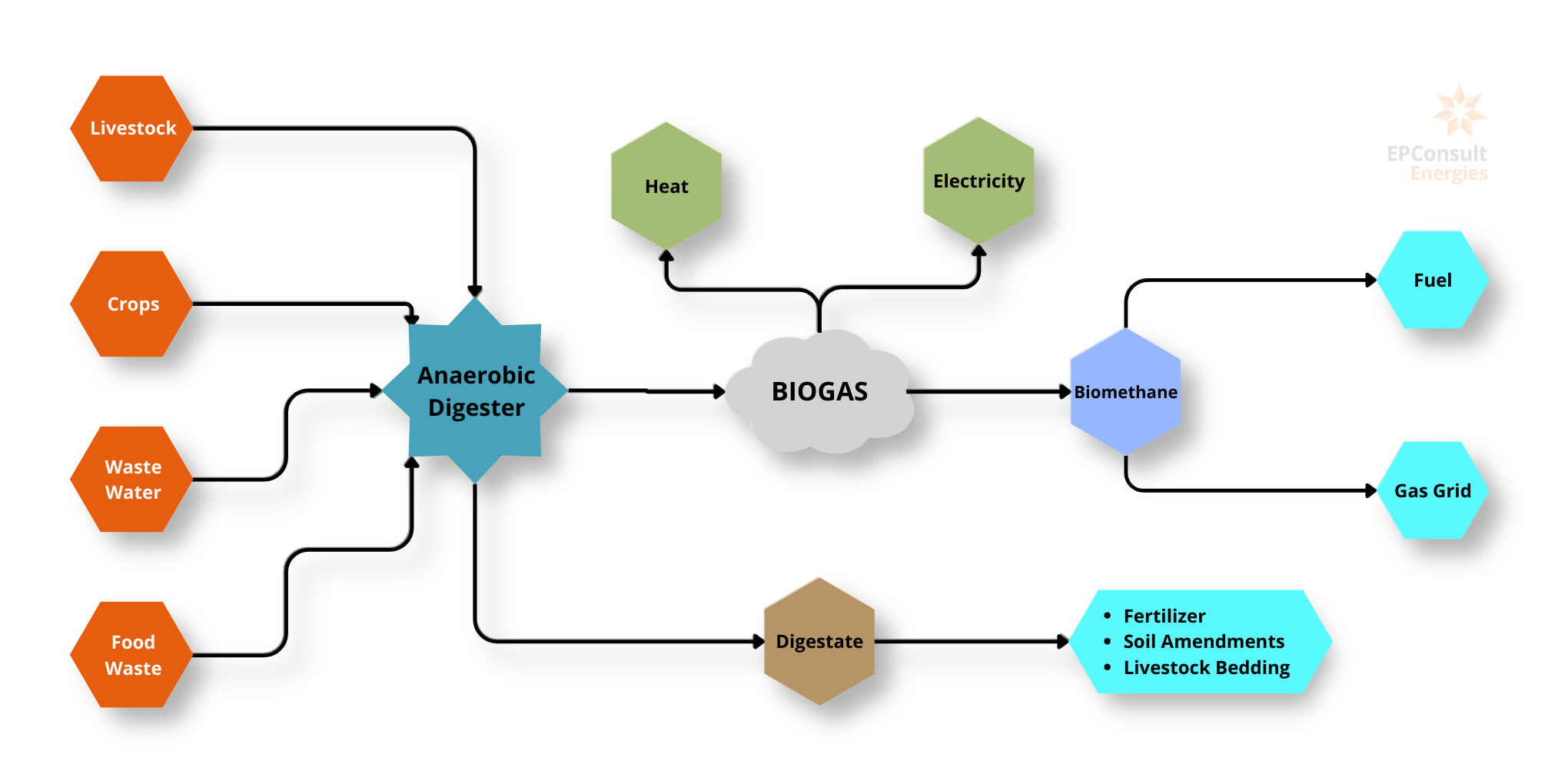
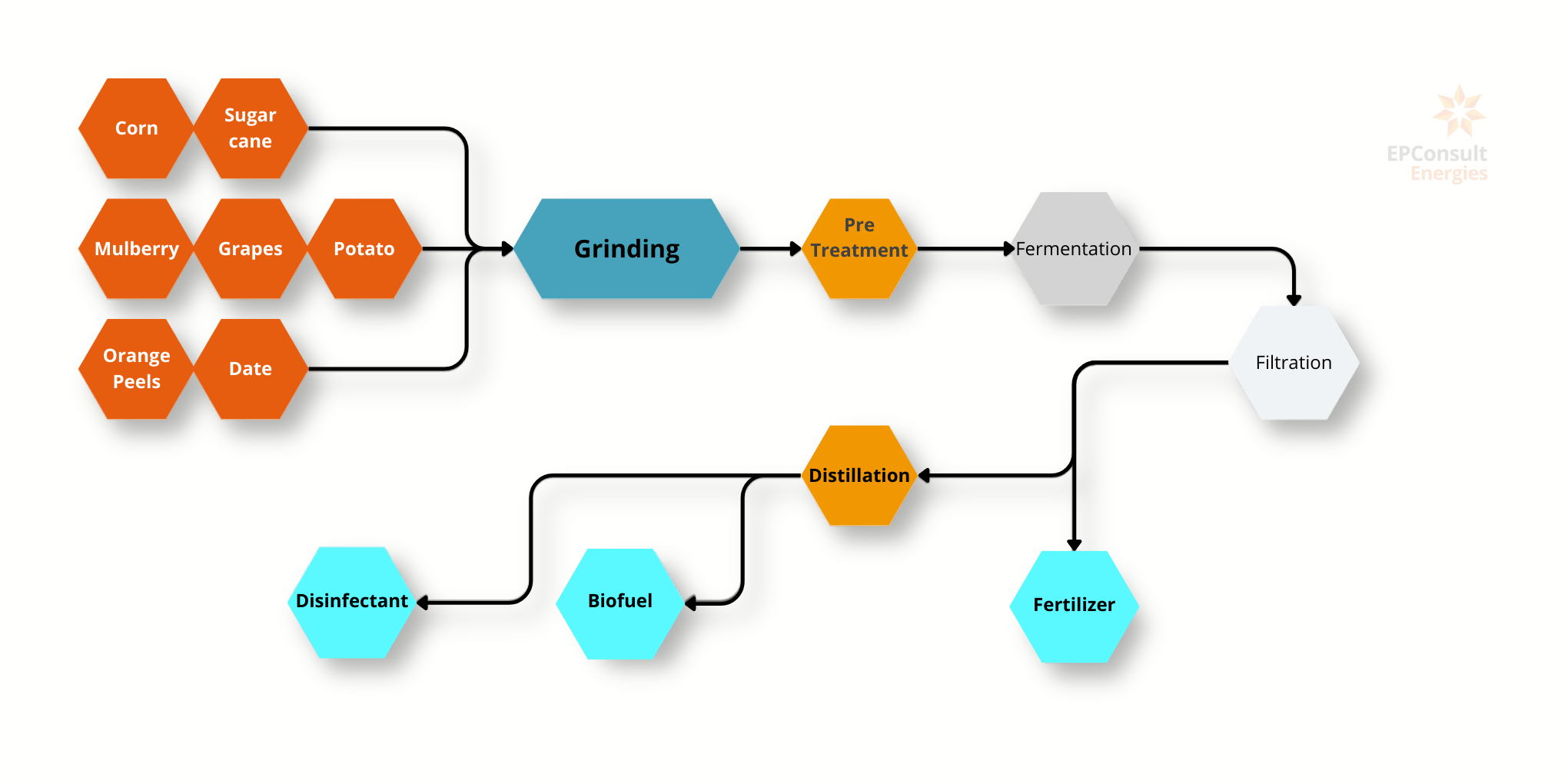 Biofuels, derived from organic materials, offer sustainable alternatives to conventional fossil fuels and are crucial in the transition to renewable energy sources. The three primary types of biofuels are biogas, bioethanol, and biodiesel, each with unique production processes, applications, and environmental benefits. Biogas is produced through anaerobic digestion of organic waste, bioethanol through fermentation of sugars in crops, and biodiesel through the transesterification of vegetable oils or animal fats. These biofuels collectively help reduce carbon emissions, promote renewable energy use, and support waste management and sustainable agricultural practices.
Biofuels, derived from organic materials, offer sustainable alternatives to conventional fossil fuels and are crucial in the transition to renewable energy sources. The three primary types of biofuels are biogas, bioethanol, and biodiesel, each with unique production processes, applications, and environmental benefits. Biogas is produced through anaerobic digestion of organic waste, bioethanol through fermentation of sugars in crops, and biodiesel through the transesterification of vegetable oils or animal fats. These biofuels collectively help reduce carbon emissions, promote renewable energy use, and support waste management and sustainable agricultural practices.
Biogas:
Production Process: Biogas is produced through anaerobic digestion of organic matter by bacteria, decomposing materials like agricultural residues, manure, and food or animal wastes in the absence of oxygen to produce methane (CH4) and carbon dioxide (CO2).
Production Costs: $0.2 to $0.3 per kilogram. Heat of Combustion (LHV): 22 MJ/kg.
Carbon Emissions: Biogas emits between 81 to 251 kg CO2e per kWh of energy produced, varying based on the feedstock and production methods. It is considered a low-carbon fuel because it recycles existing carbon in the ecosystem.
Market Dynamics:
- Production Volume: 52 million tonnes of oil equivalent (Mtoe) per year.
- Demand: Approximately 35 Mtoe per year.
Applications:
- Energy Generation: Used to generate electricity and heat, replacing natural gas in power plants and heating systems.
- Transportation: Upgraded to biomethane for use as a vehicle fuel.
- Industrial Use: Provides a renewable energy source for various industrial processes.
Waste Management: Helps reduce landfill use and associated emissions by utilising organic waste.
Bioethanol:
Production Process: Bioethanol fuel is mainly produced by the sugar fermentation. The main sources of sugar required to produce ethanol come from fuel or energy crops. These crops are grown specifically for energy use and include corn, maize and wheat crops, waste straw, willow and popular trees, sawdust, reed canary grass, cord grasses, jerusalem artichoke, myscanthus and sorghum plants.
Energy Requirement: The total energy required to produce 1 Litre of Bioethanol is 4.55 MJ.
Production Costs: 0.26-0.38 $ per kg.
Heat of Combustion (LHV): Approximately 27 MJ/kg.
Carbon Emissions: Emissions are primarily associated with the synthesis of bioethanol and vary depending on the feedstock. For example, Brazilian sugar cane ethanol produces approximately 38.5 gCO2e/MJ, while US corn ethanol produces 44.9 gCO2e/MJ.
Market Dynamics:
- Production Volume: 89 Million tonnes per year.
Applications:
- Transportation: It can be used as fuel in vehicles by mixing with gasoline at different volume fractions. Blends with smaller fractions of bio-ethanol can be used directly without any engine modifications, while higher volume fraction blends of 85% can be used only in flexible fuel vehicles (FFVs).
- Industrial Use: Serves as a solvent and in the production of chemicals.
Biodiesel
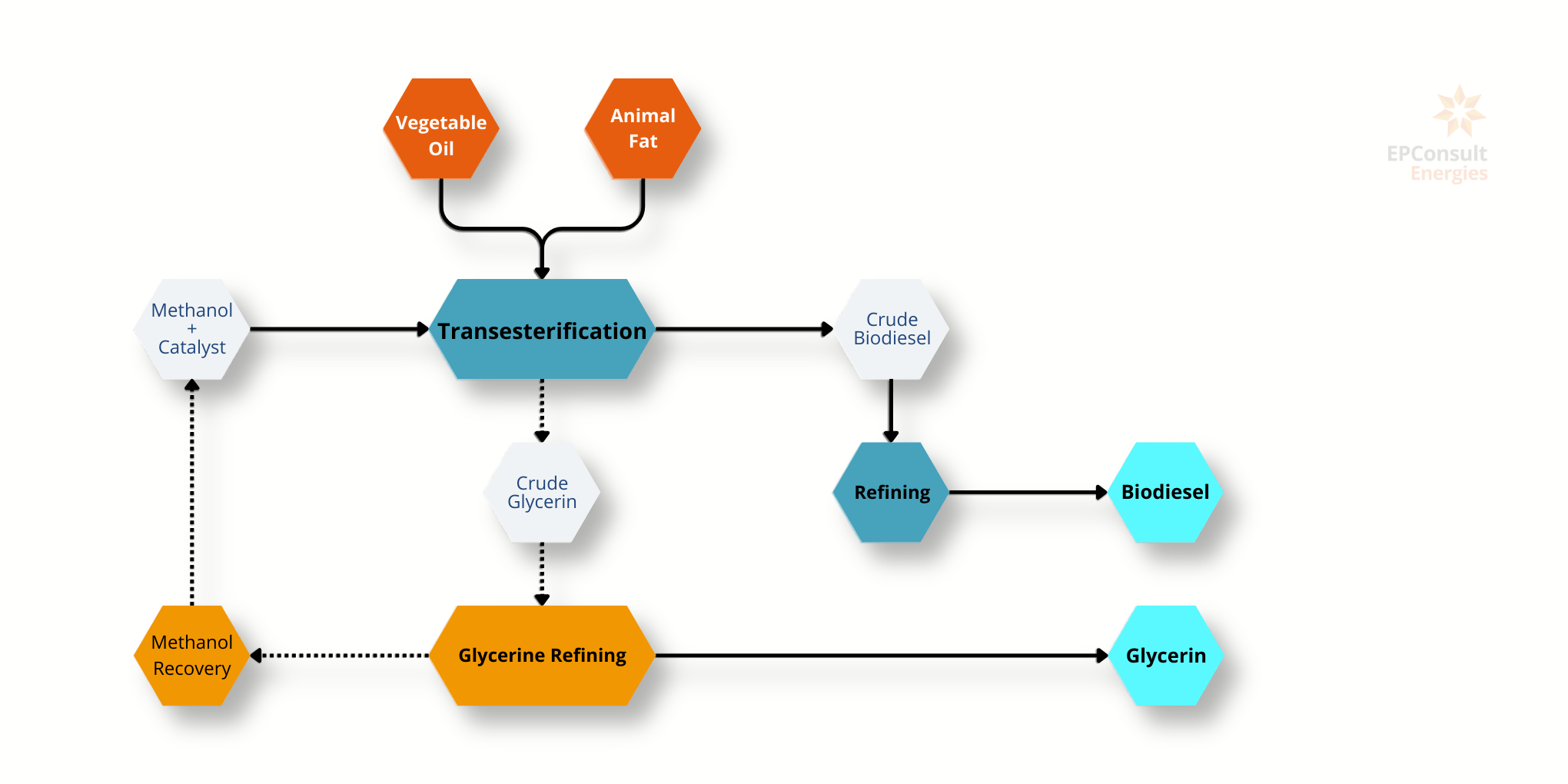 Production Process: Biodiesel is produced through the transesterification of vegetable oils or animal fats. This chemical reaction converts fats and oils into fatty acid methyl esters (FAME), which constitute biodiesel, and glycerol as a by-product.
Production Process: Biodiesel is produced through the transesterification of vegetable oils or animal fats. This chemical reaction converts fats and oils into fatty acid methyl esters (FAME), which constitute biodiesel, and glycerol as a by-product.
Energy Requirement: 0.038 GJ per Kg.
Production Costs: 1.9 $ per Kg.
Heat of Combustion (LHV): Approximately 37 MJ/kg.
Carbon Emissions: Simple combustion of biodiesel produces CO2 emissions at a rate of 5.87 pounds per gallon. Driving one mile on average emits 404 grams of CO2. Using Biodiesel can achieve 79-86% reduction in GHG emissions.
Market Dynamics:
- Production Volume: Growing, with significant contributions from the USA, Europe, and other regions. The global biodiesel market is expected to grow from $36.55 billion in 2022 to $38.93 billion in 2023 at a compound annual growth rate (CAGR) of 6.5%. The biodiesel market is expected to grow to $49.34 billion in 2027 at a CAGR of 6.1%.
- Demand: -
Applications:
- Transportation: Just about every industry can use biodiesel. The most common use is in diesel engines, which the automobile and trucking industries have naturally found the most widespread use for. Biodiesel can also be used to run agriculture machinery.
- Industrial Use: -
Challenges and Opportunities:
Feedstock Supply:
- Ensuring a consistent and sustainable supply of organic materials is critical for biofuel production.
Technological Advancements:
- Innovations in production processes can enhance efficiency and reduce costs, making biofuels more competitive with fossil fuels.
Policy and Incentives:
- Government policies and incentives are crucial for promoting biofuel adoption, including financial support and regulatory frameworks that encourage renewable energy use.
Environmental Impact:
- While biofuels generally have lower carbon footprints compared to fossil fuels, managing emissions and ensuring sustainable agricultural practices are essential to maximise their environmental benefits.
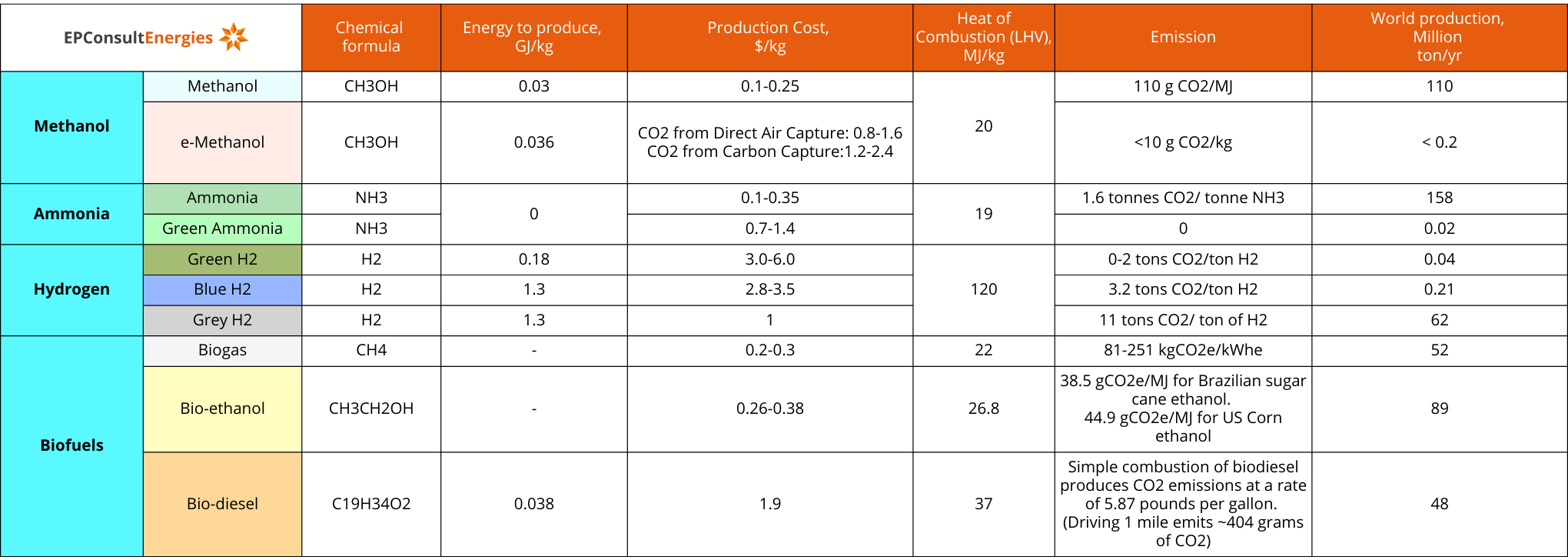
The development and adoption of various green fuels underscore the global push for sustainable energy solutions. Each fuel has distinct chemical properties, energy requirements, production costs, and environmental impacts. Below is a comparative table summarizing the key aspects of Methanol, Ammonia, Hydrogen, and Biofuels.
The table highlights the diverse landscape of green fuels, each with its own set of advantages and challenges. Methanol and its greener variant, e-Methanol, are crucial in the chemical industry, as they have significant production volumes but varying environmental impacts. Ammonia remains a vital component for fertilizers, with Green Ammonia offering a sustainable alternative. Hydrogen, particularly Green Hydrogen, presents a low-carbon solution for the future, though its production and storage remain complex and costly. Biofuels like Biogas, Bio-ethanol, and Bio-diesel offer renewable energy options with varying carbon footprints depending on the feedstock and production methods.
At EPConsult Energies, our extensive experience in the energy sector positions us as a valuable partner in navigating these challenges. With over 20 years of engineering excellence, we offer bespoke consulting services to help businesses and organisations optimise their energy strategies. Whether you're exploring the transition to green fuels or seeking to enhance your current operations, EPConsult Energies is here to provide the expertise you need.
Contact us today to learn how we can assist you in achieving your sustainability goals and advancing your energy projects.
More insights:

Hydrogen Challenges and Solutions
Hydrogen, with a history intertwined with the energy sector for over two centuries, is witnessing a remarkable resurgence, particularly as a renewable energy source in the UK's transition from fossil fuels. As the momentum around hydrogen energy grows, so does the question of its safety. While all fuels inherently pose some risks, hydrogen's unique properties, such as a wide range of flammable concentrations and lower ignition energy, necessitate additional engineering controls and careful material selection.

UK's Electric Vehicle Revolution
In a landmark decision that marked the beginning of the UK's Electric Vehicle (EV) Revolution, the UK government announced in November 2020 the cessation of sales of petrol and diesel-fueled vehicles by 2030, and hybrid vehicles by 2035. This unprecedented shift, aimed at significantly reducing the country's carbon footprint, has set the stage for a monumental transition in the transport sector, which accounted for 24% of the UK's total emissions in 2020.

Blowing Away the Carbon
There are several technical and economic reasons that offshore wind power is a viable option for decarbonizing traditional oil and gas production. Firstly, new offshore wind license areas are often located in deeper waters and at greater distances from the coast. Once land is reached, these sites are still far from potential markets. These logistical factors drive up the cost of delivering power to onshore users.

Common Myths About Solar Energy
The myths and misconceptions surrounding solar energy often need to be updated or stem from a lack of understanding of the technology. Solar energy remains efficient in less-than-ideal weather conditions, and while initial costs can be high, the long-term financial benefits are substantial. Furthermore, although the manufacturing process of solar panels does have an environmental impact, the overall effect is far less damaging than traditional energy sources.
From Coal Mines to Clean Energy
The commitment to net zero has impacted all sectors, promoting the need for low-carbon technologies. Around the UK, a vast number of decommissioned facilities are standing disused in previous industrial hubs, and this will only become more common over the coming years as more and more carbon-polluting facilities are closed. More recently, there has been research and investment on how these sites could be used to develop renewable energy sites.
Riding The Waves of Energy
The ocean is a vast playground of untapped energy. Waves, created by the wind's interaction with the water's surface, hold immense potential. Wave energy, also known as ocean wave power, aims to capture this power and convert it into usable electricity. It's like harnessing the ocean's natural heartbeat to power our world. The energy held within the world's waves is the most significant unused power source on the planet, with the total energy potential globally believed to reach a staggering 30,000 TWh per year. To put this into perspective, that's tenfold the European Union's total annual electricity usage.
- Log in to post comments
